Abstract
Background: Despite advances, the prognosis of patients with relapsed/refractory solid tumors remains poor. Recently, cancer immunotherapy has emerged as an attractive tool for the treatment of such tumors. The unique ability of NK cells to target cancer cells without antigen specificity, the ability of these cells to be used as an off-the-shelf universal donor therapy, and their better safety profile make NK cells a promising candidate for use against solid tumors. Currently, several ongoing clinical trials are evaluating NK cell therapy in solid tumors. Due to the challenges of monitoring the distribution of NK cells in humans after infusion outside of the blood, there is currently limited knowledge of the trafficking of NK cells into solid tumors. A better understanding of the homing and bio-distribution of NK cells in humans could enable the development of improved NK cell therapies and may be useful as a biomarker to assess tumor response. To date, there is no safe and effective method available to track infused NK cell therapy product in humans. We have developed a safe, non-invasive strategy to track NK cells using a submicron component of FDA approved microbubbles using ultrasound (US). These particles, termed, nanobubbles (NBs) are sub-micron gas core lipid-shell particles, measuring ~200-800 nm in diameter that can be internalized in cells which are echogenic and produce US contrast signals that are distinct from background tissue.
Objectives: To track NK cells after infusion using NB contrast with US both in vitro and in vivo (both in healthy and solid tumor mouse models).
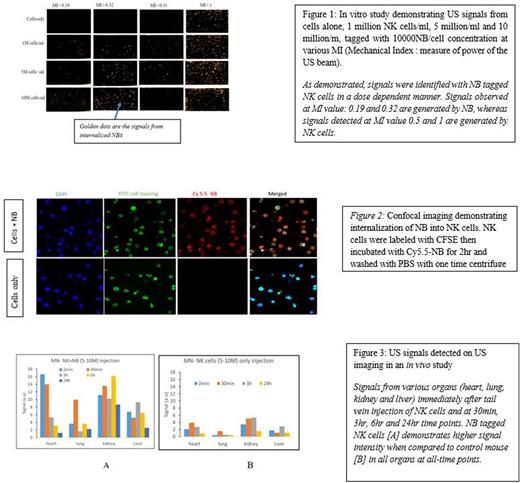
Methods:In Vitro studies: Human NK cells isolated from PBMCs of healthy donors were expanded and co-cultured with NBs at varying concentrations (5000, 10000 and 50000 NB/cell) at different time points (2hr, 4hr and 24 hr). The NB-labelled NK cells (test) and NK cells alone (control) were injected into a static/flow phantom and scanned using a Toshiba clinical ultrasound. Confocal imaging was performed to confirm the internalization of NBs into the NK cells.
In Vivo studies: Cells were prepared in a similar fashion as mentioned above with the optimized parameters obtained from in vitro studies; 10,000 NB/cell and 2 hr incubation period. NK cells were injected into the tail vein of healthy NSG immunodeficient mice and imaging of heart/lung, liver, and kidney were performed immediately after infusion, 30min, 3hr, 6hr and 24hr time points. After 24 hr, the mice were sacrificed and the organs were harvested for further analysis by flow cytometry.
Results: The in vitro studies demonstrated consistent and reproducible NB signals using US at the 2 hr time point with a 10000 NBs/cell concentration. US signals were tested and detected both in the static phantom and flow phantom (to simulate in vivo blood flow). In vivo studies in healthy mice with the injection of 5-10 million NB labelled NK cells (test) and NK cells alone (control) demonstrated robust signal from NB-labelled NK cells by US when compared to NK cells alone. The heart showed the strongest signals immediately after injection, followed by the lungs 30 minutes later, the kidney 6 hours later, and the liver 3-6 hours later. Though signals in all of these organs decreased with time, they were still easily detectable at 24 hours.
Conclusions: Our pre-clinical study demonstrates, NK cells labelled with NBs can enable a safe assessment of NK trafficking to various organs by ultrasound. Further studies will be conducted in a solid tumor (Raji lymphoma flank tumor) mouse with and without stimuli (IL-15 or chimeric antigen receptor) to alter the NK trafficking pattern to further understand the potential of this technology in the context of solid tumors.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal